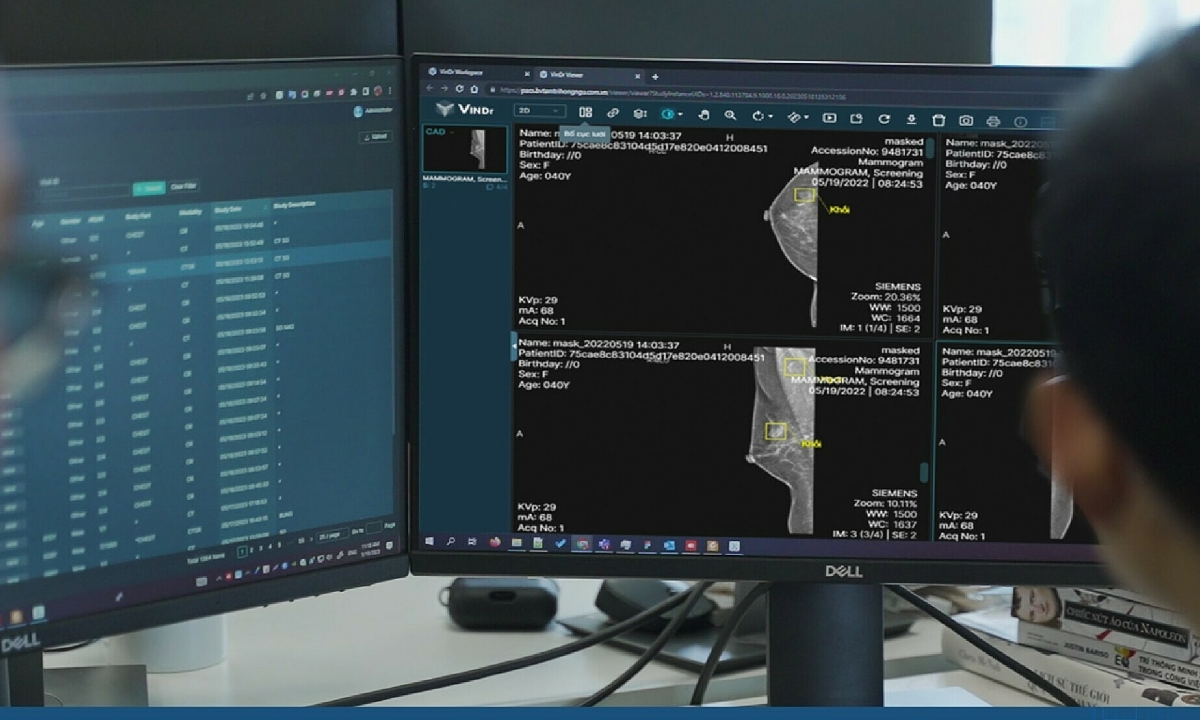
In a groundbreaking development, VinDr, an AI product developed by VinBigdata, has been granted a license by the US Food and Drug Administration (FDA) to be sold in the Vietnamese market. This makes Vietnam the 5th country to have an FDA-approved AI product for mammography diagnosis, joining the ranks of the US, France, Netherlands, and South Korea.
VinDr’s accuracy rate of 96.5% with Vietnamese data and 95.8% with American data in classifying mammograms and detecting breast cancer has made it stand out in the field of medical image analysis. The product uses advanced AI and computer vision technology to assess breast density, classify scans according to BI-RADS standards, and detect early-stage breast lesions.
Dr. Nguyen Quy Ha, Director of VinBigdata Image Analysis Technology Department, highlights the importance of AI in medical image diagnosis and early cancer screening. In Vietnam, there are limitations in early detection of cancer leading to many cases being diagnosed at a later stage. With VinDr’s help doctors can prescribe in-depth tests promptly and diagnose diseases more accurately.
Apart from mammogram analysis, VinDr also offers smart diagnostic support tools for other types of images including chest X-rays, spine X-rays